Abstract
RUVBL1, an evolutionarily conserved ATPase, is expressed in the human erythroid cytosol (Salzer et al. BBA 1999),but it has not been studied further in human red cells (RBCs). We found that more RUVBL1 is expressed in reticulocytes (RTCs) than in mature human RBCs. We prepared Western blots from the RBCs of 12 patients with RBC diseases and 7 controls (CTRs) who had no RBC anomalies. The disorders included sickle cell anemia (9), autoimmune hemolytic anemia (1), and hereditary spherocytosis (2). Blood was centrifuged, plasma and buffy coat were removed, and the RBCs were washed with PBS and re-spun. Supernatant was removed; the RBCs were boiled in a reducing buffer, electrophoresed by SDS/10% PAGE, and transferred to PVDF membranes. These were blocked in 3% nonfat dry milk and incubated with an affinity-isolated Prestige antibody to RUVBL1 from rabbit (#HPA019947, Sigma-Aldrich, validated by the Human Protein Atlas project) and anti-GAPDH ([D16H11] XP® Rabbit mAb, Cell Signaling). The blots were washed, incubated with anti-rabbit IgG (Fc), alkaline phosphatase conjugate (Promega), and protein bands were detected with Western Blue Stabilized Substrate for Alkaline Phosphatase. Relative band intensity was normalized to the intensity of GAPDH expression by scanning densitometry. RUVBL1 protein levels in the study group (SG) were compared with CTRs by an unpaired t test.
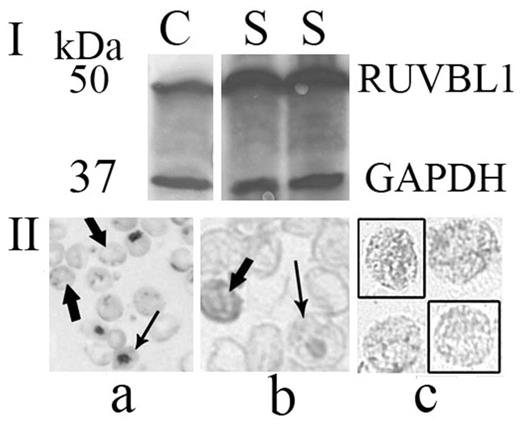
The RUVBL1 and GAPDH antibodies used each detected single bands in the human RBC samples at ~50 kDa and ~37 kDa, respectively, on Western blots, which is in accordance with what has been reported in the literature and by manufacturers. RUVBL1 levels varied between individuals and tended to be higher in the SG, which had disorders associated with high RTCs. We then stained RBCs with anti-RUVBL1. First, peripheral smears stained with methylene blue (MB) for RTCs were washed with Tris-buffered saline (TBS), processed with a Rabbit Specific HRP/DAB Detection (IHC) Kit (ab64261, Abcam), and incubated at 4o C for 2 nights with anti-RUVBL1. Second, to be sure that the RUVBL1 staining superimposed on MB wasn't artifactual, unstained RBCs were fixed in cold methanol in tubes, washed in TBS, processed with the Abcam kit, and incubated for 2 nights at 4o C with anti-RUVBL1; the protocol was completed on smears made from RBCs that had been stained with anti-RUVBL1 in the tubes. Antigen-antibody binding was detected with DAB chromogen.
The RBC preparations, evaluated on Wright-stained smears, were about 99.95% pure, with only ~0.05% white cells remaining. Analysis of 5 Western blots (Fig. I; C=CTR; S=SG) showed that the SG had 2.4-fold higher RUVBL1 levels than the CTRs (mean ± SEM, 2.434 ± 0.704, P=0.054). RTC counts were above normal in all SG patients tested (11), but they were normal in all CTRs tested (3): mean ± SEM = 281.464 ± 45.721 K/µL in the SG vs 53.900 ± 7.658 K/µL in the CTRs (P=0.027). The staining (Fig. II)shows a MB-stained RTC smear in which ribosomal RNA has been precipitated by the cationic dye (Panel a). Panel b shows MB-stained cells that were re-stained with anti-RUVBL1. The MB dye has washed out during staining; anti-RUVBL1 staining of the RBCs is variable and in the pattern of the RTCs (see arrows). The RTCs stain more intensely than the RBCs in the background. Panel c shows previously unstained RBCs that were fixed in methanol and stained with anti-RUVBL1. They show the reticulofilamentous pattern that is characteristic of RTCs. Negative controls showed no staining.
The maturation of RTCs into mature human RBCs is a complex process. Little is known about the specific molecular changes that occur during this transformation, and the possible role of RUVBL1 has not been investigated. Prior data (Salzer et al. BBA 1999) suggest a potential interaction with human RBC membrane proteins, which may be an avenue for additional study. We wish to point out that human RTCs represent an enriched source of RUVBL1 that may facilitate further analysis.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal